
The method of claim 1 wherein the PIK3CA or PTEN mutation is the H1047R, H1047L, E542K, E545K or Q546R mutation of PIK3CA.ħ.

The method of claim 4 wherein the patient has been previously treated with tamoxifen, fulvestrant, or letrozole.Ħ. The method of claim 1 wherein the hyperproliferative disorder is metastatic breast cancer, hormone receptor positive (HR+) breast cancer, HER2 expressing breast cancer, or estrogen receptor positive (ER+) breast cancer.ĥ. The method of claim 1 wherein the patient is administered a therapeutically effective amount of the combination of GDC-0032 and a chemotherapeutic agent.Ĥ. The method of claim 1 wherein the patient is administered a therapeutically effective amount of GDC-0032 as a single agent.ģ. A method of treating a hyperproliferative disorder in a patient comprising administering a therapeutically effective amount of GDC-0032 as a single agent or a combination of GDC-0032 and a chemotherapeutic agent selected from 5-FU, docetaxel, eribulin, gemcitabine, GDC-0973, GDC-0623, paclitaxel, tamoxifen, fulvestrant, dexamethasone, pertuzumab, trastuzumab emtansine, trastuzumab and letrozole to the patient, wherein a biological sample obtained from the patient, prior to administration to the patient of GDC-0032 as a single agent or the combination of GDC-0032 and the chemotherapeutic agent, has been tested for PIK3CA or PTEN mutation status, and wherein PIK3CA or PTEN mutation status is indicative of therapeutic responsiveness by the patient to GDC-0032 as a single agent or the combination of GDC-0032 and the chemotherapeutic agent.Ģ. Method for producing a vesicle dispersionĬomposition and method for etiological treatment and prevention of diseases and/or complications associated with chronic glucose metabolism destabilizationġ. Mq and t-propyl siloxane resins compositions Nanocapsule encapsulation system and method Tea Tree Oil and Benzoyl Peroxide Acne Treatment White silver-containing wound care device Inclusion of clemizole in future anti-HCV cocktails can represent an attractive paradigm for increasing current virologic response rates.Method for treating an environment that may be or is contaminated with an undesirable bacterial, virus and or sporeĬombination of amines and vanadium (IV)/(V) compounds for the treatment and/or prevention of diabetes mellitus Clemizole can yield high-level synergy with the protease inhibitor class. Finally, no cross-resistance to clemizole of SCH503034-resistant mutants (or vice versa) was observed. Furthermore, combination of clemizole with SCH503034 decreased the frequency of drug-resistant mutants, compared with treatment with either drug alone. In contrast, combinations of clemizole with either interferon, ribavirin, or the nucleoside (NM283) and nonnucleoside (HCV796) HCV polymerase inhibitors were additive. Clemizole's antiviral effect was highly synergistic with the HCV protease inhibitors SCH503034 and VX950, without toxicity. Data were analyzed using Loewe additivity and Bliss independence models for synergy, and resistance studies were performed using HCV colony formation assays. Luciferase reporter-linked HCV replication assays were used to study the antiviral effects of drug combinations that included clemizole. We hypothesized that the combination of clemizole with other anti-HCV agents can increase the antiviral effect over that achieved with each drug alone and could also decrease the emergence of viral resistance.
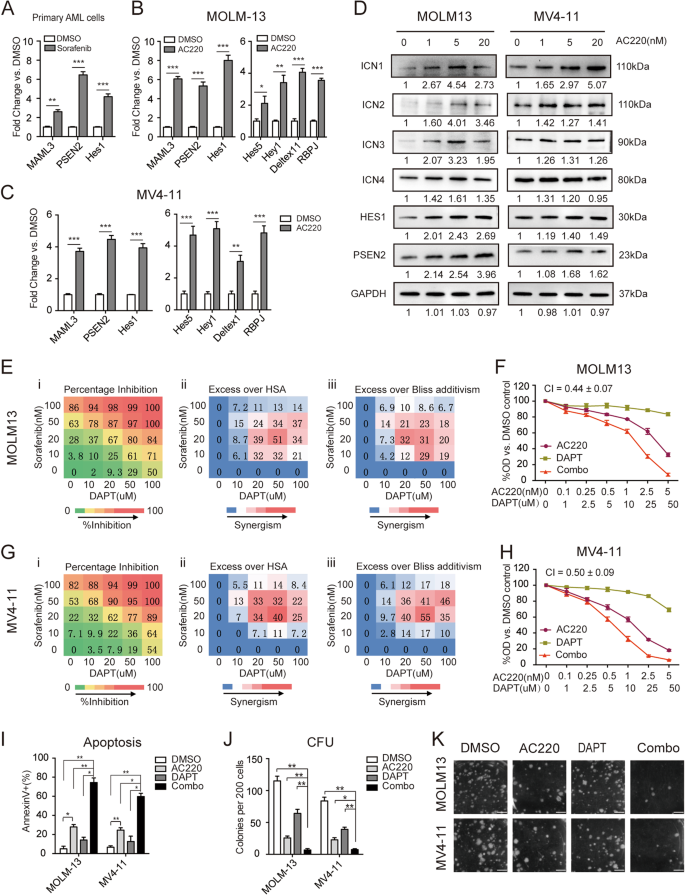
Although significant, clemizole's antiviral effect is moderate (50% effective concentration of 8 μM against an HCV genotype 2a clone). We recently identified a compound, clemizole hydrochloride, that inhibits NS4B's RNA binding and hepatitis C virus (HCV) replication.


 0 kommentar(er)
0 kommentar(er)
